Sure! Here’s a fresh, heartwarming retelling of the science story:
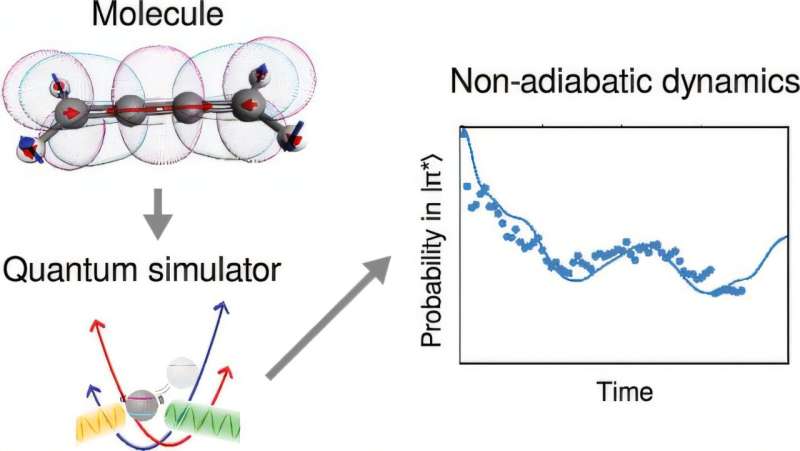
Imagine a world where tiny molecules leap into action when they absorb light, undergoing incredible transformations in a flash. This fascinating process underlies many natural and technological wonders, from the way plants grow to how medicine fights disease. Now, a team of Australian researchers has taken a huge step in understanding these lightning-fast changes by using a quantum computer in a groundbreaking way.
When light hits a molecule, something magical happens. Electrons jump up energy levels, atoms start to vibrate, and bonds between atoms wobble—all within a blink of an eye. These changes influence everything from how plants photosynthesize to the functioning of solar panels and even innovative cancer therapies.
Yet, simulating these swift quantum phenomena has posed a challenge for traditional computers. They find it tough to keep up, needing massive computational resources to make sense of these microsecond rapid changes. Enter quantum computers, which embody quantum behavior by their very nature and could pave the way for accurate simulations.
Dancing with Ions
In their research, the team harnessed a trapped-ion quantum computer, a remarkable device that keeps ions suspended in a vacuum using electromagnetic fields. Normally, quantum computers work with bits of information known as qubits, but to accurately model molecular behavior, the researchers employed a unique technique that integrates vibrational modes of atoms—called “bosonic modes.”
This innovative mixed qudit-boson simulation significantly simplifies the requirements for quantum computing power, making it possible to simulate molecules much more efficiently. The researchers delved into how specific molecules, such as allene, butatriene, and pyrazine, behave post-light absorption. Each of these molecules displays complex dances of electronic and vibrational interactions, making them perfect candidates for this ambitious experiment.
Here’s the exciting part: they were able to slow down molecular interactions by a staggering factor of 100 billion! While the real interactions only last femtoseconds, the simulation stretched them out into milliseconds, allowing researchers to observe each intricate step.
A Leap Forward
What sets this study apart is not just what they simulated, but how they did it. Traditional quantum simulations of similar complexity require extensive resources—think 11 qubits and about 300,000 operations, far beyond what current tech can handle. In contrast, this team achieved their results with a single ion and a single laser pulse, making their approach at least a million times more efficient than previous methods!
The team also dabbled in “open-system” dynamics, examining how molecules interact with their surroundings. By deliberately introducing controlled noise to the ion’s environment, they replicated the energy loss experienced in real molecular interactions, demonstrating the capability of quantum simulations to account for environmental variables—an often tricky problem for classical computers.
The Road Ahead
This pioneering work shines a light on the future of quantum chemistry. Although today’s quantum computers might seem limited, the findings reveal that even smaller, well-designed systems can tackle significant scientific questions. Understanding the real-world behavior of atoms and molecules is crucial for breakthroughs in materials science, medicine, and energy solutions.
The researchers are optimistic that with just a bit more scaling—they envision 20 or 30 ions—quantum simulations could tackle chemical systems currently deemed too complex for traditional supercomputers. Imagine the endless possibilities this could unlock in our quest to understand the very building blocks of life!
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()
If you would like to see similar science posts like this, click here & share this article with your friends!

