A research team led by Charles McCrory from the University of Michigan, alongside labs from UC Davis and UCLA, has created a way to capture carbon dioxide and transform it into metal oxalates. These can then serve as ingredients for making cement.
The findings are detailed in a study published in the journal Advanced Energy Materials.
“This research illustrates how we can utilize a waste product, carbon dioxide, and convert it into something valuable,” McCrory, an associate professor of chemistry and macromolecular science, explained. “Rather than just burying carbon dioxide, we’re repurposing it into something useful.”
This project was part of the Center for Closing the Carbon Cycle (4C), an Energy Frontier Research Center led by researchers at UC Irvine. One major aim of 4C is to find methods for capturing carbon dioxide and turning it into useful fuels and materials.
The traditional method to make cement, typically using limestone and calcium silicates, comes with a high energy cost and substantial carbon emissions. McCrory and his team are exploring how carbon dioxide can be converted into materials suitable for creating alternative kinds of cement.
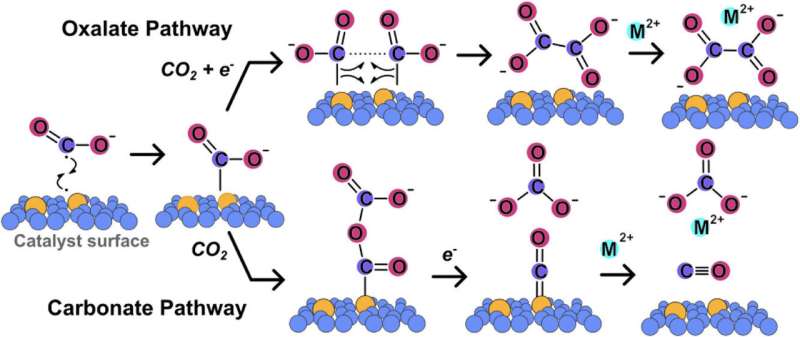
Metal oxalates, which are basic salts, can serve as viable substitutes for traditional cement. While lead is known to act as a catalyst for converting carbon dioxide into these salts, its extensive use poses environmental and health concerns.
The 4C team has found a solution by using polymers to modify the environment around the lead catalysts, drastically reducing the amount of lead required to a trace level—similar to what’s found in commercial porous materials.
McCrory focuses on optimizing the chemical environment around catalyst sites to enhance their efficiency. His team’s work demonstrates that by fine-tuning this environment, they can significantly lessen the lead needed in the reaction that turns carbon dioxide into oxalates.
The process involves two electrodes: one helps convert carbon dioxide into oxalate, while the other is a metal electrode reacting to release metal ions that bind with the oxalate, forming solid metal oxalates.
“Those metal ions combine with oxalate to create a solid, which separates from the solution,” McCrory explained. “This is the final product that can be integrated into the cement-making process.”
Co-lead author Anastassia Alexandrova from UCLA added that their calculations support the mechanism’s viability. “Typically, catalysts are often discovered unpredictably, and effective industrial mixes can be complex. In this situation, we see a trace lead impurity unexpectedly acting as a catalyst, which might suggest other similar opportunities in catalyst discovery,” she said.
McCrory emphasized that once carbon dioxide is transformed into solid metal oxalates, it won’t re-enter the atmosphere under normal conditions. “It’s a genuine capture process since it turns into a solid, and that solid has practical, valuable applications,” he noted.
He believes they are on the path to developing a scalable method for creating these products. Current work focuses on expanding the electrolysis process for carbon dioxide, with future studies aimed at scaling up the solid product creation.
“We’re not there yet, but we see potential for scalability,” McCrory reflected. “Reducing lead usage to trace amounts helps address the environmental concerns associated with large-scale operations.”
More information:
Rowan S. Brower et al, Selective Electrochemical Reduction of CO2 to Metal Oxalates in Nonaqueous Solutions Using Trace Metal Pb on Carbon Supports Enhanced by a Tailored Microenvironment, Advanced Energy Materials (2025). DOI: 10.1002/aenm.202501286
If you would like to see similar Tech posts like this, click here & share this article with your friends!

