The potential of nickel
Developing materials for batteries is akin to baking: Mix the right components, apply heat, and yield something new. For batteries, scientists are on the lookout for materials to create positive and negative electrodes (cathodes and anodes, respectively). Positive electrodes operate by accepting ions and electrons to generate electrical flow used in devices such as flashlights, laptops, cell phones, and vehicle and data center systems.
As the demand for nickel-rich devices rises, researchers are continuously seeking materials that store more energy and have longer lifetimes. Standard lithium-ion batteries are constrained by cost and energy storage capacity, according to Xiao. To cut costs, more affordable materials like nickel and manganese are often blended with cobalt in battery production.
Recent studies, including those by Xiao’s team at PNNL, have focused on the cost-effective integration of higher nickel levels into battery cathodes. Nickel can store more energy than cobalt, making materials more cost-effective. Nickel may also aid in lowering the expenses related to scaling up cathode production.
Despite its advantages, working with nickel presents challenges, Xiao noted. Nickel-rich lithium cathode materials tend to form agglomerations called “polycrystals,” akin to a cookie filled with chocolate chips. The boundaries between crystals weaken as the battery charges and discharges, leading to cracking over time, compromising battery performance and lifespan.
“Think of all those tiny particles clumped together and moving around as the battery cycles,” Xiao explained. “These movements can create cracks, which can harm the battery.”
Over the past five years, Xiao and her team have searched for materials forming single-crystal structures, resembling a plain chocolate chip cookie with evenly distributed chocolate. These single crystals are less vulnerable than polycrystal structures, offering hope to eliminate major challenges in nickel-rich cathode materials.
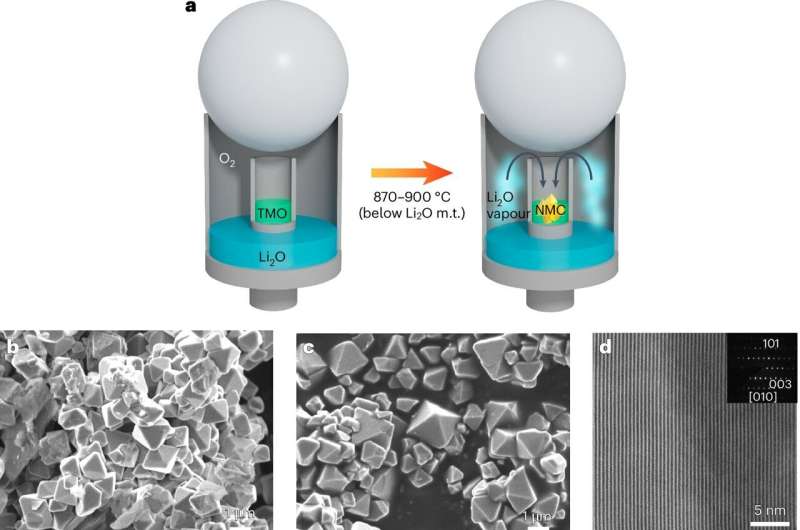
The anomaly of sublimation
In recent years, Xiao’s team explored various lithium salts provided by industry partner Albemarle Corporation. Mixing these salt ingredients, or precursors, with nickel-rich precursors generates cathode material. A common production method is to melt lithium salt, which then reacts with the nickel-rich precursor. Researchers typically prefer lithium hydroxide (LiOH) for this process due to its low melting point.
Li2O, on the other hand, has a high melting point at 1,438 degrees Celsius and is rarely used in cathode material synthesis. However, experiments at Xiao’s materials synthesis lab at PNNL revealed an unexpected result: Combining the nickel-rich precursor with Li2O around 900 degrees Celsius led to the rapid formation of single-crystal cathode material.
Iterating the reaction multiple times, Xiao and her team sought to understand the mechanism. Ultimately, they engaged their industry partner Thermo Fisher Scientific, which examined the reaction using a MicroReactor instrument. Observations from this collaboration and a new experiment design unveiled the sublimation phenomenon.
“We are thrilled to observe the reaction between Li2O and the precursor under the microscope,” said Libor Novák, creator of the MicroReactor at Thermo Fisher Scientific.
The latest research confirms that the mechanism is driven by Li2O sublimation. In baking terms, this is akin to blending cookie dough with vaporized chocolate, resulting in a chocolate cookie without distinct chocolate chunks in it when cut in half.
“The vapor can permeate everywhere, including the pores or surface of other precursors, and immediately trigger a reaction,” Xiao explained. “Single crystals form much faster in the presence of those vapors.”
The team applied the Li2O sublimation phenomenon to directly convert spent polycrystals into single crystals through a simple mixing-and-heating process. The successful production of new single crystals indicates that Li2O significantly simplifies the recycling process of used polycrystals. Production line scraps can be efficiently transformed into high-performance single crystals using this salt ingredient, Xiao explained.
Moreover, the new single crystals, whether from fresh precursors or spent polycrystals, showed stability for up to 1,000 charge/discharge cycles.
Potential benefits for manufacturing
The discovery of Li2O-derived single crystals offers a new approach to producing single crystals due to time and energy savings, as well as high performance. However, further research is required before batteries can be manufactured, Xiao cautioned. The current commercial use of Li2O is costly as it’s not widely utilized in material synthesis. Nonetheless, Xiao emphasized that Li2O can be easily produced from processing other lithium salts like LiOH.
With industry partners, Xiao and her team are striving to scale up the process at reduced manufacturing costs. They anticipate providing insights to strategic partners by 2026.
More information:
Bingbin Wu et al, Unusual Li2O sublimation promotes single-crystal growth and sintering, Nature Energy (2025). DOI: 10.1038/s41560-025-01738-4

