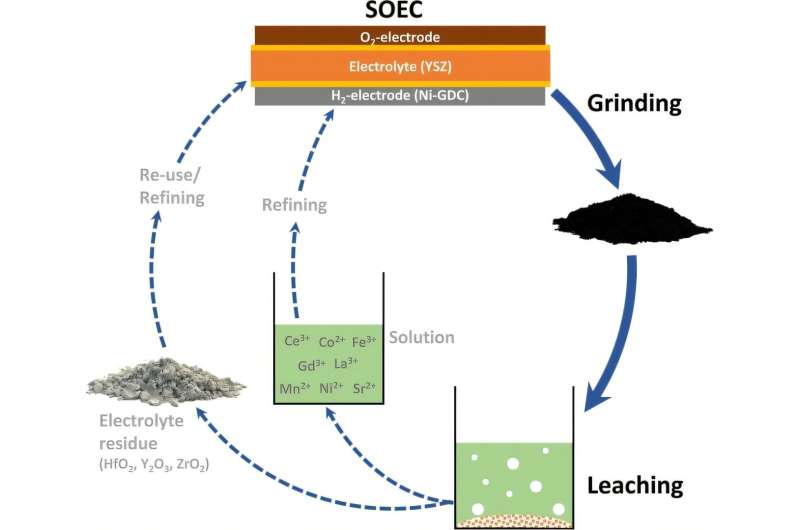
Hydrogen-producing electrolysis cells are made with rare earth metals, which often end up discarded as waste once the cells are no longer usable. Researchers at TU Bergakademie Freiberg are exploring ways to extract and recycle these materials from old electrolysis cells, allowing them to be reused in new ones. This study has been detailed in the Journal of Sustainable Metallurgy.
With the increasing focus on hydrogen energy, rare earth metals like scandium, lanthanum, and cerium have become highly valued. A single module of solid oxide electrolysis cells can contain up to 150 kilograms of these precious metals for every 10 megawatts produced.
The latest findings from the TU Bergakademie Freiberg team indicate that these metals can be effectively extracted from the electrolysis electrodes using hydrometallurgical techniques, allowing them to be reused instead of relying on raw materials.
The researchers have successfully tested their recycling method at a small scale, with experiments involving just 0.2 grams of cell material per trial. “We’re currently working to scale up our findings for larger tests of up to 50 grams,” said Dr. Pit Völs, one of the researchers involved.

Eco-friendly Metal Processing
The research team has emphasized environmentally friendly hydrometallurgical recycling techniques, especially focusing on leaching to dissolve metals into a liquid solution. “Initially, we mechanically separate the electrode and electrolyte materials from the steel casing of the cells,” Dr. Völs explained. “Then, using acids, we leach out the rare earth metals from the electrodes, which are the focus of our study.”
Subsequently, the metals will be isolated using environmentally friendly chemicals and recycled. This novel recycling strategy will also undergo evaluation through a simulation-based life cycle assessment.
Transforming Waste into Resources
The findings are part of the GrInHy3.0 project, conducted alongside industry partners. The goal of the project, as described by Professor Alexandros Charitos of TU Bergakademie Freiberg, is to create a process for producing hydrogen in solid oxide electrolysis cells. “This technology aims to loop recycled metals back into the materials cycle, ultimately reducing the environmental impact associated with future waste generated during hydrogen production,” he added.
Over the next three years, the technology will be tested in real-world conditions at facilities operated by project partners, such as electrolyzer manufacturer Sunfire SE and steelmaker Salzgitter Flachstahl GmbH. This plant is expected to enable the production of 14 kilograms of hydrogen every hour.
More information:
Pit Völs et al, Leaching of Solid Oxide Electrolyzer Cells for a Circular Hydrogen Economy, Journal of Sustainable Metallurgy (2025). DOI: 10.1007/s40831-025-01080-9
If you would like to see similar Tech posts like this, click here & share this article with your friends!

