
Texas engineers have found a new discovery in modern batteries that could enhance their life cycles.
As batteries age, they tend to perform less efficiently, resulting in the need for more frequent recharging, such as in the case of smartphones. This degradation is partly caused by a thin film that forms on the metal anode during charging and discharging. While this film has its benefits, its rough texture gradually wears down the battery.
Researchers have identified a temporary version of this film, known as the transient solid-electrolyte interphase (T-SEI), which appears during rapid discharge speeds and dissolves back into the battery once the process is complete. This temporary film promotes a smoother surface on the metal anode than the permanent version, providing protection without causing abrasion and long-term damage.
Published in the Proceedings of the National Academy of Sciences, this research has the potential to significantly improve the performance and safety of devices like smartphones and increase the viability of batteries for large-scale energy storage.
“By managing this temporary interphase, we can develop batteries that perform better under high-demand conditions, last longer, and are less likely to fail,” said Stephen T. Fuller, a Ph.D. student at the Cockrell School of Engineering’s McKetta Department of Chemical Engineering and lead author of the study.
Battery research usually focuses on the charging process when an external power source transfers electrons from the cathode to the anode to power the battery. This new discovery stems from a detailed examination of the discharge process, suggesting that further breakthroughs are possible in this area.
“Prior to my time at UT, I concentrated on the recharging process, but I was interested in studying the discharge process, which has been largely overlooked in our community,” said Kent Zheng, an assistant professor of chemical engineering at UT. He was established as a Forbes’ 30 Under 30 in Science honoree. “Our project involved the use of an aqueous battery with an electrolyte solution primarily consisting of water to facilitate charging and discharging by shuttling ions through the battery.”
-
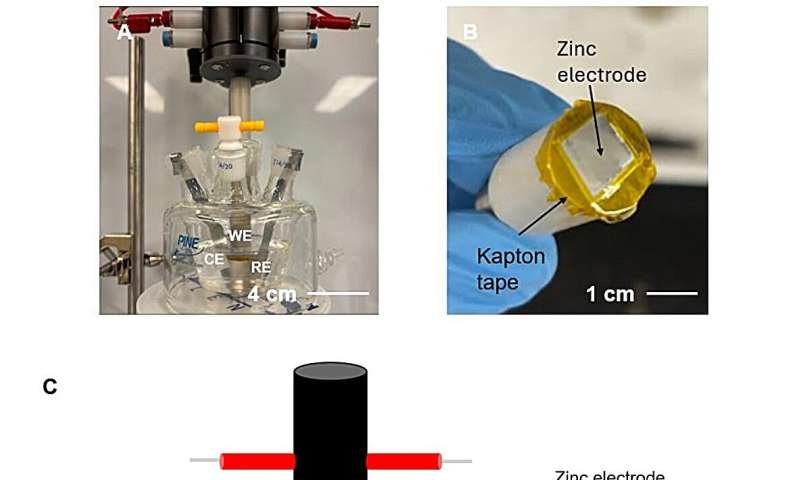
RDE cell used for in-operando visualization. Credit: Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2425752122
-
Credit: University of Texas at Austin
Aqueous batteries have the potential for large-scale energy storage, allowing the storage of renewable energy sources like wind and solar energy until needed. This type of battery is less flammable and uses more sustainable materials, but further development is required before large-scale implementation.
Advanced electroanalytical systems, including a rotating disk electrode and in operando visualization, were crucial in understanding the formation and behavior of T-SEI. The film forms due to supersaturation, resulting in salt deposition on the electrode.
When the battery is at rest, the transient interface completely dissolves, leaving a clean, flat surface. The T-SEI layer formation during cycling reduced surface roughness by 42%, leading to improved battery efficiency and longevity.
The researchers aim to apply this discovery to other battery types to assess the presence of T-SEI. Utilizing this phenomenon could help mitigate the formation of dendrites, irregular structures that develop over multiple charge-discharge cycles, potentially shortening battery life or diminishing performance over time.
More information:
Stephen T. Fuller et al, On the hidden transient interphase in metal anodes: Dynamic precipitation controls electrochemical interfaces in batteries, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2425752122

