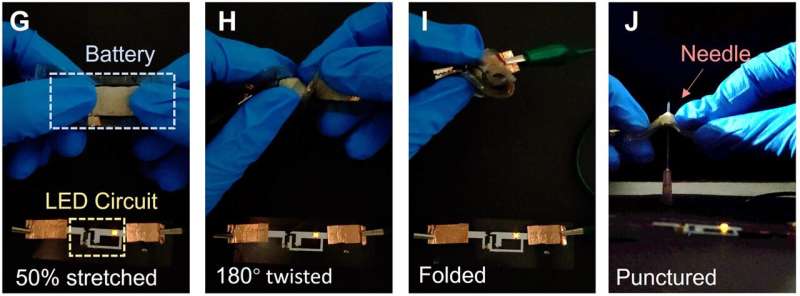
A group of researchers at the University of California, Berkeley, the Georgia Institute of Technology and the Hong Kong University of Science and Technology has created a flexible, self-repairing lithium battery that stays stable after 500 charge/discharge cycles. In their study published in the journal Science Advances, the team explains how they developed the battery and its potential applications.
In recent years, scientists have been working on batteries for various uses. One type of battery being explored is the stretchable battery, which could be integrated into wearable technology. Recently, researchers at Linköping University announced the creation of a , expanding its potential applications. In this new research, the team at UC Berkeley developed a that can also repair itself.
To create the battery, the research team started with a zwitterionic polymer that had both a positive and . These polymers allow water molecules to bond with the charged parts and lithium ions to be attracted by the negative parts of the plastic. This structure keeps water tightly bound within the battery, reducing the risk of splitting when voltage is applied, while still releasing lithium ions as needed.
Next, the team introduced an as a cross-linker and a fluorine-free Li salt-based hydrogel electrolyte with a stability window of up to 3.11 volts. This design enabled the battery to extract water from the air, further decreasing the chance of water molecule splitting under electrical stress.
