Imagine transforming ordinary materials into extraordinary ones using just light! A recent breakthrough in polymer chemistry unveils a heartwarming story of innovation, sustainability, and teamwork that could change the way we think about materials in our daily lives. Join us as we explore this exciting journey in the world of polymers.
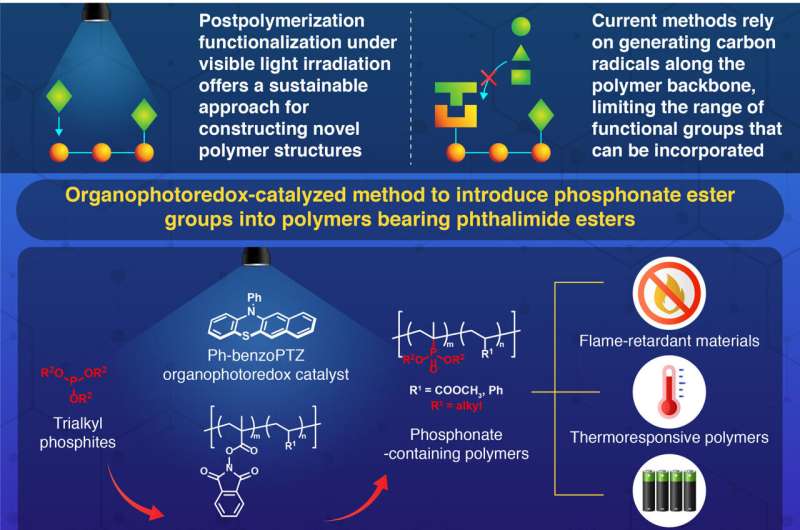
With a growing demand for advanced materials, scientists are constantly on the lookout for innovative ways to enhance polymers—those versatile substances found in everything from packaging to medical devices. One exciting method gaining traction is called post-functionalization, which allows researchers to tweak existing polymers to add new properties after they’ve been made.
Traditionally, this process required generating unstable radicals, which limited the types of modifications possible. But a talented team of researchers, led by Professor Shinsuke Inagi from the Institute of Science Tokyo, has turned the game around. Their method not only allows for new modifications but does so in a way that’s gentle on the environment—using blue LED light and an organophotoredox catalyst.
In a recent study published in Angewandte Chemie International Edition, Inagi, along with former graduate student Tomohiro Tamano and Professor Hirohisa Ohmiya from Kyoto University, shared an exciting new way to introduce phosphonate esters into polymer chains. This achievement opens the door to a richer variety of polymers with unique functionalities.
The innovative technique relies on something called radical–polar crossover (RPC) chemistry. At its core, it starts with creating a carbocation on the polymer backbone, which can react with various chemical groups. “What we’re showing here is truly groundbreaking,” explains Professor Inagi, highlighting the method’s potential to pave the way for new, tailorable polymer architectures that were previously impossible.
Let’s break down how it works: The researchers set the stage by using poly(methacrylate) derivatives containing a special phthalimide ester group. When exposed to blue LED light, the organophotoredox catalyst gets to work, generating a radical that eventually transforms into a positively charged intermediate structure on the polymer chain.
This exciting transformation allows the polymer to bond with nucleophiles, such as trialkyl phosphites, incorporating beneficial phosphonate groups into its structure. The result? A unique polymer blend featuring diethyl isopropenylphosphonate that not only defies traditional polymer creation methods but also shows exceptional fire resistance and temperature responsiveness.
Why does this matter? As it turns out, even a modest addition of phosphonate groups can significantly enhance fire resistance, making these polymers invaluable for applications in flame-retardant materials and safety-focused technologies like lithium-ion batteries. These advancements may help prevent battery fires, ensuring greater safety in our everyday devices.
As the team looks ahead, they’re eager to explore how this innovative strategy can enable the incorporation of other useful chemical groups into polymers. It’s a thrilling prospect that indicates a brighter, greener future for materials science!
More information:
Tomohiro Tamano et al, Organophotoredox‐Catalyzed Postfunctionalization of Poly(methacrylate) Derivatives via Radical–Polar Crossover Phosphonylation, Angewandte Chemie International Edition (2025). DOI: 10.1002/anie.202507572
If you would like to see similar science posts like this, click here & share this article with your friends!


